Introduction:
Advancements in the treatment of multiple myeloma (MM) with proteasome inhibitors [bortezomib (bort), carfilzomib (carf)], monoclonal antibodies [daratumumab (dara), isatuximab (isa) and elotozumab (elo)], and immunomodulatory drugs [IMiD) [lenalidomide and pomalidomide (pom)] have improved outcomes. However, using lenalidomide as frontline treatment and maintenance therapy exposes greater numbers of patients to lenalidomide prior to relapse. Consequently, the treatment of lenalidomide-refractory (LR) disease is an increasing area of concern. Given the lack of direct comparisons of different treatment regimens for LR MM, we used a systematic review to identify randomized controlled trials (RCTs) that included subgroup analysis of LR patients. The objective of our study was to compare the efficacy of novel treatment regimens in randomized trials for patients with LR MM using a network meta-analysis.
Methods:
Three databases were searched: MEDLINE/PubMed, Embase, and Cochrane Registry of Controlled Trials. All studies published between January 1, 2005 and December 30, 2019 were queried using keywords "multiple myeloma" and "randomized controlled trial". Only RCT's were included. Primary outcome was progression free survival (PFS) of patients enrolled in myeloma RCT's. Two independent investigators reviewed all the studies and resolved any conflict through mutual discussion.
WNetwork meta-analysis using random-effects model was performed to generate direct and indirect comparisons between various treatment groups. Frequentist method was used to rank the interventions and P-score was generated. A higher P score (close to 1.00) corresponded to superior progression free survival. A P-value of <0.05 was considered statistically significant. Hazard ratios (HR) with 95% confidence interval (CI) were calculated. I2statistic was used to assess heterogeneity between the studies as defined by the Cochrane Handbook for Systematic Reviews with a value >50% considered as significant heterogeneity. We used 'R' (Bell Labs, Murray Hill, NJ, USA) for generating our statistical analysis and plots.
Results:
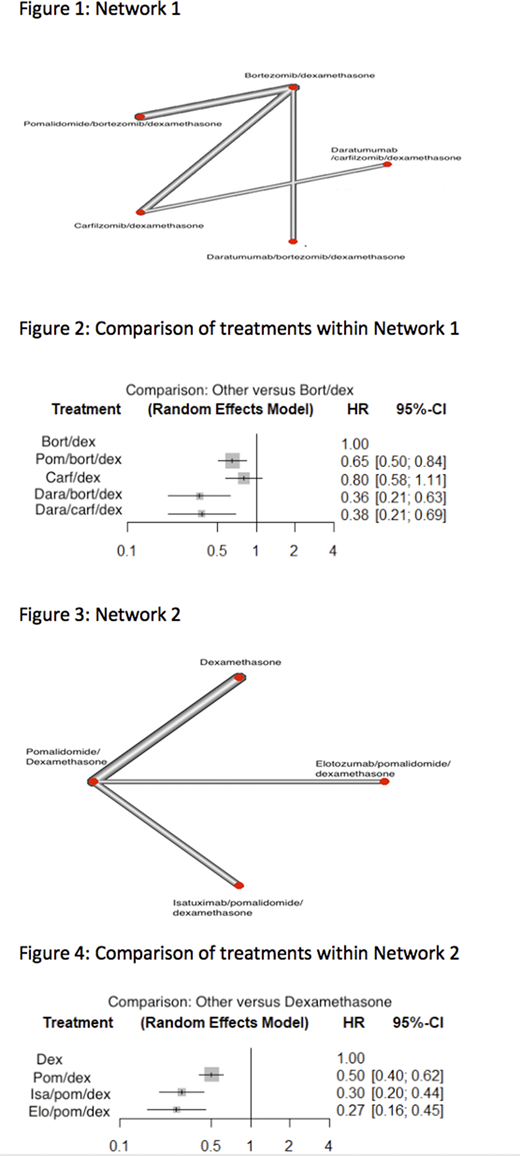
Our initial search strategy yielded 1171 results. After excluding duplicates, trials in progress, subset analysis and non-randomized studies, 123 RCTs were identified. Only 8 studies clearly reported outcomes of patients with LR myeloma and 7 studies (1698 patients) were included in final analysis of two discrete networks. Pom/bort/dex (HR: 0.65,95% CI: 0.50-0.84), dara/bort/dex (HR: 0.36, 95% CI: 0.21-0.63), and dara/carf/dex (HR: 0.38, 95% CI: 0.21-0.69) were all found to have improved PFS compared to bort/dex (Figure 2). These interventions were ranked as follows: Dara-bort-dex was the most efficacious with a P-score of 0.8758, followed by Dara/carf/Dex (P of 0.8514), Pom/bort/dex (P of 0.4791), Carf/dex (P of 0.2707) and Bort/dex (P of 0.0231).
Within Network 2 (Figure 3), pom/dex (HR :0.50, 95% CI= 0.40-0.62), isa/pom/dex (HR: 0.30, 95% CI= 0.20-0.44) and elo/pom/dex (HR: 0.27, 95% CI=0.16-0.45), were all found to have improved PFS compared to dexamethasone (Figure 4).
In Network 2, the ranking of interventions was as follows with Elo-pom-dex (P of 0.8716) the most efficacious, followed by Isa-pom-Dex (P of 0.7932) and Pom-dex (P of 0.3352).
Conclusion:
The results of our network meta-analysis of 1698 patients with lenalidomide refractory myeloma enrolled in seven randomized myeloma trials demonstrate that for lenalidomide refractory myeloma, triplet therapy is superior to doublet therapy. Regimens with the highest efficacy in this subset of patients were triplets containing monoclonal antibodies (such as dara/elo/isa). There is a need for further randomized data to better ascertain the best standard of care for these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal